Rare-Earth-Catalyzed Regiodivergent Hydrosilylation of Aryl Alkenes
Wufeng Chen#, Ni Zhang#, Zhengqi Chai, Junnian Wei, Gen Luo* and Wen-Xiong Zhang*
ACS Catal. 2024, 14, XXXXX–XXXXX. ( #Authors contributed equally)
https://doi.org/10.1021/acscatal.3c05747
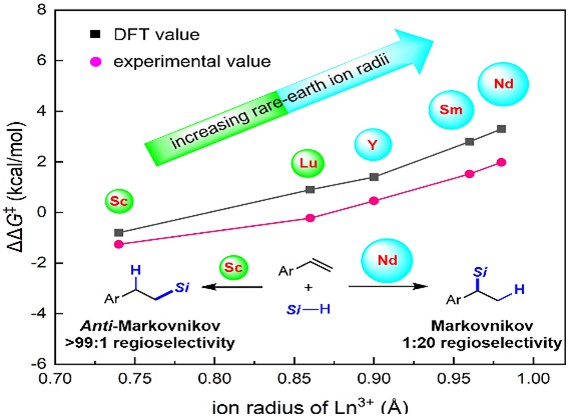
While transition metal-catalysts have shown the ability to regulate the Markovnikov or anti-Markovnikov regioselective hydrosilylation of aryl alkenes, the selective control of anti-Markovnikov hydrosilylation of aryl alkenes is still a huge challenge in rare-earth catalyst systems. In this study, we report the rare-earth-catalyzed regiodivergent hydrosilylation of aryl alkenes. Specifically, we achieved the highly regioselective anti-Markovnikov hydrosilylation of aryl alkenes with a scandium alkyl complex Cp*AmtBuScCH2SiMe3 (Cp* = pentamethylcyclopentadienyl, AmtBu = tBuNC(Me)NtBu, tBu = t-butyl) as a catalyst. Two key intermediates, e.g. the scandium hydride and scandium phenethyl complex for anti-Markovnikov hydrosilylation, were characterized. Guided by Density Functional Theory (DFT) calculations, we successfully achieved the selective inversion of aryl alkenes in Markovnikov hydrosilylation using a neodymium halide complex [Cp*AmiPrNdCl]2 (AmiPr = iPrNC(Me)NiPr, iPr = isopropyl) with the larger ion radius and reduced steric hindrance in conjunction with LiCH2SiMe3. Interestingly, our study has demonstrated the significant influence of gradually increasing rare-earth ion radii on controlling the increasing Markovnikov selectivity of hydrosilylation reactions, possibly due to the enlargement of the coordination space around rare-earth metal ions. Furthermore, through a comparison of computational and experimental data, we have observed a high level of consistency, reaffirming the potential of using calculations to predict experimental outcomes and providing researchers with valuable insights.




